Introduction
Graft-versus-host disease (GvHD) remains a crucial complication associated with significant morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT). High-dose post-transplantation cyclophosphamide (PTCy) targets alloreactive donor T cells proliferating early after HSCT, promotes regulatory T cells, and prevents severe GVHD. The effectiveness of PTCy-based regimens varies depending on the graft source and intensity of the preparative regimen. Improvement of GVHD control with novel targeted agents, added to the backbone of PTCy, could further increase the safety of HSCT and reduce the morbidity and complications of standard regimens of pharmacological immune suppression. In this pilot trial, we evaluated the safety and efficacy of abatacept and vedolizumab in unmanipulated haploidentical transplantation among children with leukemia.
Materials and methods
A total of 54 pts with acute leukemia (AML- 18, ALL-36, 26 female, 28 male, the median age at HSCT - 7,4 years (0,4-16,8), underwent unmanipulated allogeneic bone marrow (BM) (n=53) or peripheral blood stem cell (PBSC)(n=1) transplantation between March 2022 and May 2023. All donors were haploidentical relatives, represented Disease status at transplant was CR1 in 25 pts, CR³2 in 29 pts. For all patients, it was the 1-st transplantation. All patients received myeloablative conditioning, either TBI-based (n=30) or 24 treosulfan-based (n=24), with either cyclophosphamide (n=47) or etoposide (n=7) as a second agent. Prophylaxis of graft-versus-host-disease (GVHD) consisted of CsA from day-1 to day 180, 50 mg/kg of Cph on days +3 and +4, abatacept at 10 mg/kg on days +5, +14, +28, +45, +60 and vedolizumab at 10 mg/kg (capped at 300 mg) on days -1, +14, +28. The median dose of CD34+ cells was 4,7 x10 6/kg (range 1,2-17), and the CD3 dose was 42 x10 6/kg.
Results
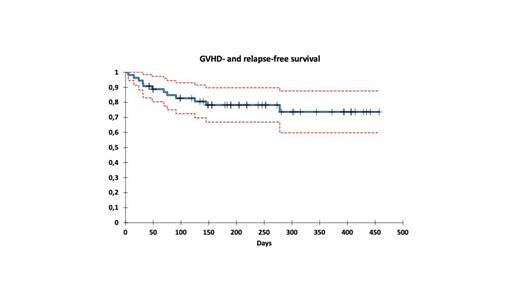
Primary engraftment was achieved in 53 (98%) pts; the median time to neutrophil and platelet recovery was 20 and 23 days, respectively. All engrafted pts had verified morphologic remission and achieved complete donor chimerism by day +30. One patient failed to engraft, relapsed, and required rescue with a second HSCT from an alternative donor. The regimen was generally well tolerated. Acute toxicity consisted of grade III-IV mucositis (n=5); grade II-III hemorrhagic cystitis (n=4); grade III-V veno-occlusive disease (n=2) (all cases of VOD developed after pre-HSCT use of inotozumab); bloodstream infections (n=8). Four patients were admitted to the intensive care unit due to septic events or VOD. The median follow-up time for survivors was seven months (range: 1 -15). Transplant-related mortality was 2% (95% CI:0,3-14): one patient with ALL previously treated with inotozumab died in CR due to consequences of severe VOD on day + 60. The cumulative incidence of aGVHD grade II-IV was 36% (95% CI, 25 - 52), and grade 3-4 was 9% (95% CI, 4 - 22). In 13 cases, aGVHD was limited to skin: grade 2 (n=12), grade 4 (n=1), in two cases to the gut: grade 3(n=1), grade 4(n=1), three patients had skin and gut GVHD: grade 2 (n=2), grade 3(n=1) and one had skin and liver GVHD grade 3. The cumulative incidence of gut aGVHD grade II-IV was 9% (95% CI, 4 - 22). Eleven patients with GVHD responded to first-line immunosuppressive treatment, while 8 received additional therapy. The cumulative incidence of relapse was 11% (95%CI:5-26) in the entire cohort. EFS and OS were 89% (95%CI: 79-97) and 98% (95%CI: 94-100). The GVHD- and relapse-free survival was 78% (95%CI:67-88).
Conclusion
Our data suggest that adding vedolizumab and abatacept to the PTCy-based GVHD prophylaxis backbone is well tolerated and may reduce the incidence of gut GVHD among children with acute leukemia grafted from haploidentical donors. This approach can be further developed to reduce exposure to calcineurin inhibitors and deploy additional anti-leukemia effectors after HSCT.
OffLabel Disclosure:
Balashov:Octafarm: Other: Lecturers fee. Maschan:Miltenyi Biotec: Honoraria.
vedolizumab as GVHD prophylaxis

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal